As an example, here we discuss implant-associated infection from the mechanics point of view on bacteria adhesion affected by the biomaterial surface roughness:
1. Implant-associated infection, a leading cause of failure in many biomedical devices, is caused by adhesion of bacteria to the surface of biomaterials. Implant-associated infection is difficult to treat, for example, joint replacement infections may occur deep around the artificial implants.
2. Microbial adhesion and subsequent biofilm formation are mediated by van der Waals attractive forces, electrostatic repulsive forces, and surface hydrophobicity. The predominance of these forces is dependent on the distance between the microorganism and the surface, usually at distances greater than 50 nm van der Waals forces are the main factor, while at closer distances (10−20 nm) a combination of both van der Waals forces and electrostatic interactions controls cell adhesion.
3. Implant surface roughness is an important property relevant for the bacterial adhesion process, with the irregularities of the surfaces normally promoting bacterial adhesion and colonization.
4. For metals used in medical implants, the desired surface roughness is usually below 10 nm. At nanometre scale rough surface promotes friction, hence reduces the mobility of the bacteria; this sessile environment expedites the biofilm growth.
5. To reduce the roughness, surface nanotopography of medical implants is an important approach that can control the extent of bacterial adhesion.
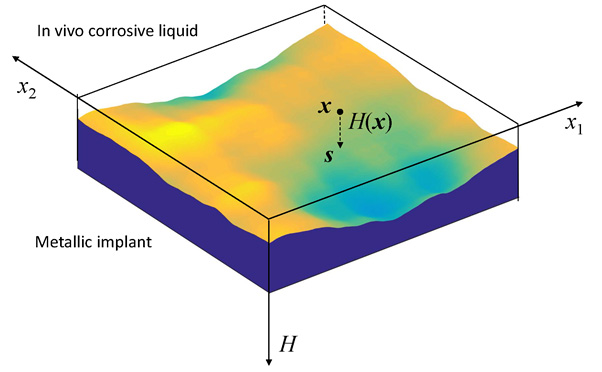
6. Surrounding the implant are soft and hard tissues, as well as the corrosive body liquid. Whether the roughness of an initially nanoscale smooth surface will grow or decay is of crucial importance for implant devices.
7. For a stress metal implant under shallow chemical etching, the roughness with spatial frequency below a critical value grows while the roughness of higher frequency decays.
8. A rougher surface had lower constraint for electrons to escape from peaks, resulting in lower chemical potential.
References:
Tan, H. (2016) In vivo surface roughness evolution of a stressed metallic implant. Journal of the Mechanics and Physics of Solids, 95, 430–440.
Truong, V.K., Pham, V.T.H., Medvedev, A., Lapovok, R., Estrin, Y., Lowe, T.C., Baulin, V., Boshkovikj, V., Fluke, C.J., Crawford, R.J., Ivanova, E.P. (2015) Self-organised nanoarchitecture of titanium surfaces influences the attachment of Staphylococcus aureus and Pseudomonas aeruginosa bacteria. Appl. Microbiol. Biotechnol. 99, 6831-6840.
Ryu, J.J., Shrotriya, P. (2015) Mechanical load assisted dissolution response of biomedical cobalt-chromium and titanium metallic alloys: Influence of in-plane stress and chemical environment. Wear 332-333, 662-668.
Desrousseaux, C., Cueff, R., Aumeran, C., Garrait, G., Mailhot-Jensen, B., Traoré, O., Sautou, V. (2015) Fabrication of acrylonitrile-butadiene-styrene nanostructures with anodic alumina oxide templates, characterization and biofilm development test for Staphylococcus epidermidis. PLoS ONE 10, Article number e0135632.
Yoda, I., Koseki, H., Tomita, M., Shida, T., Horiuchi, H., Sakoda, H., Osaki, M. (2014) Effect of surface roughness of biomaterials on Staphylococcus epidermidis adhesion. BMC Microbiology 14, 234-240.
Wang, J., Yao, J., Gao, H. (2012) Specific adhesion of a soft elastic body on a wavy surface. Theoretical and Applied Mechanics Letters, Article 014002.